A new research project to identify biomarkers for the CMT2A
26 marzo 2024
A new research project is underway, supported by our association and carried out by the team of Professor Stefania Corti of the Dino Ferrari Centre/University of Milan (Doctors Rizzo, Abati and Anastasia).
The project aims to identify (or with other pathologies due to mutation of Mitofusin 2), the presence, in the blood of patients with CMT2A, of possible biomarkers of disease that may be useful to diagnose and/or control the development of pathology.
What are biomarkers?
A biomarker is a biological signal (such as a DNA sequence or a protein) whose presence in the body is linked to a certain disease. To be valid, however, it must be possible to measure it accurately, reliably and quickly.
Why are they important for the CMT2A?
Recent research has shown that patients with Charcot-Marie-Tooth disease have higher levels of certain proteins in their blood serum (however, these findings need further confirmation, and so far no specific study has investigated the serum of patients with CMT2A).
THE SCIENTIFIC STUDY
Biomarkers in the blood are gaining more and more popularity as affordable and low-cost tools to guide diagnosis and control the development of a disease.
In the case of CMT, they can play an important role because these diseases have a slow clinical progression that can make it difficult to observe changes in the short term. In addition, they could help to better clarify the mechanisms responsible for the disease.
No study has already evaluated blood biomarkers in CMT2A because research on blood has so far concentrated on the most common subtype (CMT1A). This is the first one.
Overall, with many new therapies close to testing, it is crucial to find molecular biomarkers through which you can evaluate the effectiveness of treatments on this slowly progressing disease.
Research purpose
The aim of this project, therefore, is to investigate potential serous biomarkers of CMT2A in order to find a method (low cost and easily accessible) to make diagnoses and to evaluate therapeutic efficacy in studies.
Experimental plan
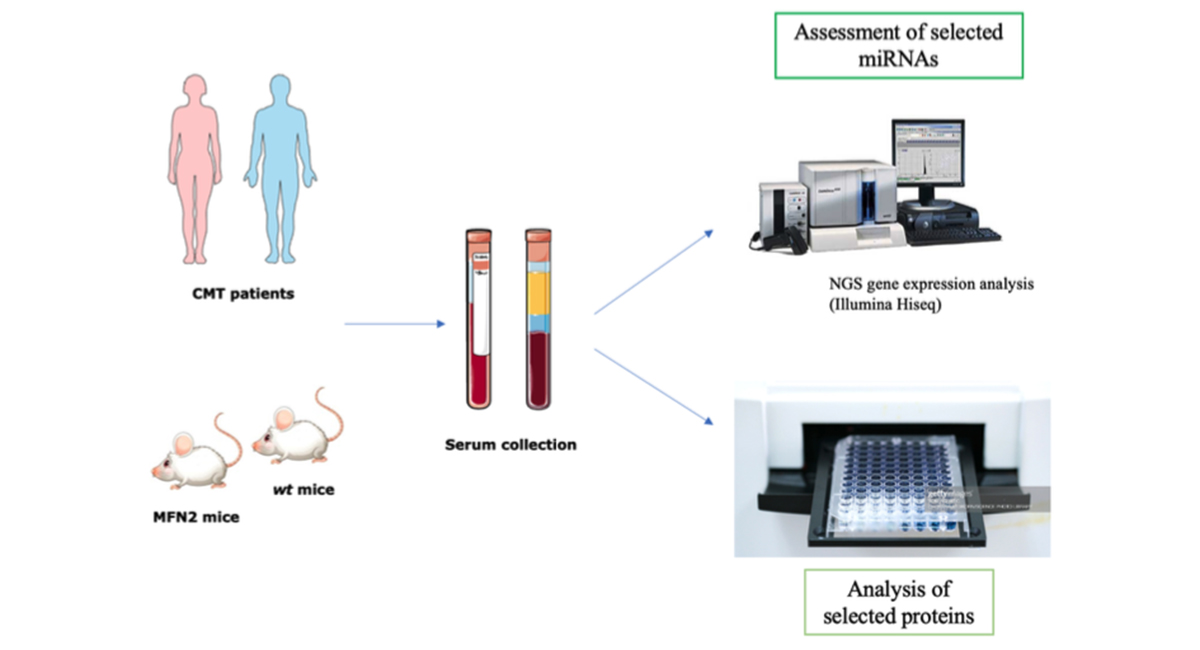
Experimental research consists of 3 steps:
- Collection of serum from patients with CMT2A and mouse models
- Evaluation of serum proteins selected by enzymatic immunosorbent test (ELISA)
- Evaluation of microRNAs selected by real-time PCR. Circulating myrrh will be isolated from the same serum using a special kit.
Who can participate in the study?
Currently limited only to Italy.
For more info, you should contact Doctor Claudia Alberti at claudia.alberti@unimi.it